Psittacosis
This article needs to be updated. (July 2014) |
| Psittacosis | |
|---|---|
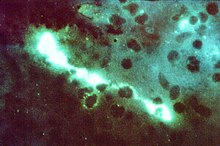 | |
| Direct fluorescent antibody stain of a mouse brain impression smear showing C. psittaci | |
| Specialty | Infectious medicine Pulmonology |
Psittacosis—also known as parrot fever, and ornithosis—is a zoonotic infectious disease in humans caused by a bacterium called Chlamydia psittaci and contracted from infected parrots, such as macaws, cockatiels, and budgerigars, and from pigeons, sparrows, ducks, hens, gulls and many other species of birds. The incidence of infection in canaries and finches is believed to be lower than in psittacine birds.
In certain contexts, the word is used when the disease is carried by any species of birds belonging to the family Psittacidae, whereas ornithosis is used when other birds carry the disease.[1]
In humans
[edit]Signs and symptoms
[edit]In humans, psittacosis typically presents as a flu-like illness with an incubation period of 5–19 days. The severity of the disease varies, ranging from asymptomatic cases to systemic illness with severe pneumonia. Early symptoms often mimic typhoid fever and include high fevers, chills, headache, muscle aches, joint pain, diarrhea, conjunctivitis, nosebleeds, and a reduced white blood cell count. Some patients develop pink, blanching maculopapular eruptions known as Horder’s spots, resembling the rose spots seen in typhoid fever. Spleen enlargement is common toward the end of the first week, and psittacosis may then progress into a serious lung infection.[2]
As the disease advances, it primarily presents as an atypical pneumonia, with a persistent dry cough, shortness of breath, and fatigue. Severe cases can lead to extensive lung involvement, as seen in X-rays showing patchy infiltrates or diffuse whiteout of lung fields. Headache can be intense enough to resemble meningitis, sometimes accompanied by nuchal rigidity. In rare cases, stupor or coma may develop. Complications, though uncommon, include myocarditis, encephalitis, endocarditis, liver inflammation, joint inflammation, and keratoconjunctivitis. Severe pneumonia may require intensive-care support, though fatal cases remain rare, occurring in less than 1% of infections.[2]
Transmission route
[edit]Humans contract psittacosis through inhalation of airborne particles from dried bird excreta, feathers, or respiratory secretions. Less commonly, direct contact with infected birds or contaminated materials may also lead to infection. The bacteria can survive in the environment for extended periods, increasing the risk of indirect transmission.
Birds, especially parrots, cockatiels, pigeons, and poultry, can carry Chlamydia psittaci without showing symptoms, but stressed or immunocompromised birds may develop respiratory distress, lethargy, and weight loss. Infected birds may intermittently shed the bacteria, even if they appear healthy.[2]
Diagnosis
[edit]Diagnosing psittacosis can be challenging due to its non-specific symptoms, requiring a combination of clinical evaluation, laboratory tests, and exposure history. Blood analysis typically shows a normal white blood cell count, though marked leukocytosis can occasionally occur. Liver enzymes are abnormal in about half of the patients, with mild elevations in aspartate transaminase. Inflammatory markers, such as erythrocyte sedimentation rate and C-reactive protein, can be significantly elevated. Differential diagnosis must distinguish psittacosis from typhus, typhoid fever, and atypical pneumonia caused by Mycoplasma, Legionella, or Coxiella burnetii (Q fever).[2]
Laboratory tests used for diagnosis include:
- Polymerase Chain Reaction (PCR): Detects bacterial DNA from respiratory samples and is considered highly specific.
- Serologic Testing: Identifies a fourfold or greater increase in antibody titers against Chlamydia psittaci in blood samples, supporting diagnosis when combined with clinical presentation.
- Culture: Although C. psittaci can be isolated from respiratory secretions, culture is rarely performed due to biosafety risks and should only be conducted in specialized laboratories.
- Chest X-rays: May reveal lung inflammation, patchy infiltrates, or diffuse pneumonia in severe cases.
- Bronchoalveolar Lavage (BAL): In some cases, characteristic inclusions known as "Leventhal-Cole-Lillie bodies" can be observed within macrophages in BAL fluid.
Given the hazards of culturing C. psittaci, PCR and serologic testing remain the preferred diagnostic methods, while exposure history plays a crucial role in confirming suspected cases. [2]
Treatment
[edit]Psittacosis is effectively treated with antibiotics, primarily tetracyclines:
- Doxycycline (first-line treatment, typically prescribed for 10–14 days).
- Macrolides (e.g., azithromycin) may be used for patients who cannot tolerate tetracyclines.
- Early treatment reduces the risk of severe complications and hospitalization.
If left untreated, the disease can become severe, leading to respiratory failure or multi-organ involvement.[3]
Prevention and Control
[edit]To minimize the risk of psittacosis, several preventive measures should be taken. Proper hygiene must be maintained when handling birds or cleaning cages to reduce contamination. Infected birds should be isolated and treated promptly to prevent the spread of infection. Regular veterinary check-ups are essential to monitor pet birds for signs of illness. Individuals working in high-risk environments, such as pet stores or poultry farms, should use protective equipment like masks and gloves. Public health surveillance and control measures play a crucial role in preventing outbreaks. Additionally, avoiding exposure to sick or wild birds, particularly in high-risk areas, further reduces the chances of infection.
Epidemiology
[edit]Psittacosis cases occur worldwide, with sporadic outbreaks reported in both developed and developing countries. Occupational exposure among bird breeders, poultry workers, and veterinarians increases the risk of infection.
Surveillance data from the CDC indicate that reported human cases have declined due to improved diagnostic capabilities and awareness, though under-reporting remains a concern. In some regions, outbreaks have been associated with imported pet birds or poorly regulated bird trade markets.[4][5]
History
[edit]Psittacosis was first documented in the 1870s, with major outbreaks occurring in the early 20th century. A significant outbreak in 1929–1930, linked to imported parrots, led to increased scientific interest and research into the disease. The causative agent, Chlamydia psittaci, was identified in the 1930s, paving the way for improved diagnostic techniques and treatment options.
Since then, regulations on the pet trade and public health interventions have contributed to reducing the spread of psittacosis, though sporadic outbreaks continue to occur.
Recent Research and Emerging Strains
[edit]Recent studies have identified genetic variations in C. psittaci, suggesting potential differences in virulence and host specificity. Advances in molecular diagnostics, such as next-generation sequencing, are improving our understanding of psittacosis epidemiology and transmission dynamics.
Researchers are also investigating the potential for antibiotic resistance in C. psittaci, though current treatment protocols remain effective. Ongoing studies aim to develop vaccines to protect both birds and humans from infection, which could help mitigate outbreaks in high-risk populations.
In birds
[edit]
In birds, Chlamydia psittaci infection is referred to as avian chlamydiosis. Infected birds shed the bacteria through feces and nasal discharges, which can remain infectious for several months. Many strains remain quiescent in birds until activated under stress. Birds are excellent, highly mobile vectors for the distribution of chlamydial infection because they feed on, and have access to, the detritus of infected animals of all sorts.
Signs
[edit]C. psittaci in birds is often systemic and infections can be inapparent, severe, acute, or chronic with intermittent shedding. Signs in birds include "inflamed eyes, difficulty in breathing, watery droppings, and green urates."[6]
Diagnosis
[edit]Initial diagnosis may be by symptoms, but is usually confirmed by an antigen and antibody test. A polymerase chain reaction-based test is also available. Although any of these tests can confirm psittacosis, false negatives are possible, so a combination of clinical and laboratory tests is recommended before giving the bird a clean bill of health.[6]
Epidemiology
[edit]Infection is usually by the droppings of another infected bird, though it can also be transmitted by feathers and eggs,[7] and is typically either inhaled or ingested.[6]
C. psittaci strains in birds infect mucosal epithelial cells and macrophages of the respiratory tract. Septicaemia eventually develops and the bacteria become localized in epithelial cells and macrophages of most organs, conjunctiva, and gastrointestinal tract. It can also be passed in the eggs. Stress commonly triggers onset of severe symptoms, resulting in rapid deterioration and death. C. psittaci strains are similar in virulence, grow readily in cell culture, have 16S-rRNA genes that differ by <0.8%, and belong to eight known serovars. All should be considered to be readily transmissible to humans.[citation needed]
C. psittaci serovar A is endemic among psittacine birds and has caused sporadic zoonotic disease in humans, other mammals, and tortoises. Serovar B is endemic among pigeons, has been isolated from turkeys, and has also been identified as the cause of abortion in a dairy herd. Serovars C and D are occupational hazards for slaughterhouse workers and for people in contact with birds. Serovar E isolates (known as Cal-10, MP, or MN) have been obtained from a variety of avian hosts worldwide, and although they were associated with the 1920s–1930s outbreak in humans, a specific reservoir for serovar E has not been identified. The M56 and WC serovars were isolated during outbreaks in mammals.
Use as a biological weapon
[edit]Psittacosis was one of more than a dozen agents that the United States researched as potential biological weapons before the nation suspended its biological weapons program.[8]
Notable casualties
[edit]In 1930, during the 1929–1930 psittacosis pandemic, Lena Rose Pepperdine died of parrot fever. She was the first wife of George Pepperdine, the founder of Pepperdine University.[9]
References
[edit]- The initial content for this article was adapted from sources available at https://www.cdc.gov.
- ^ "ornithosis" at Dorland's Medical Dictionary[dead link]
- ^ a b c d e Australian Guidelines for the Prevention and Control of Infection in Healthcare (PDF). National Health and Medical Research Council. May 2019. p. 274. ISBN 978-1-86496-028-0. Archived from the original (PDF) on 14 May 2020. Retrieved 23 January 2020.
- ^ Gregory DW, Schaffner W (1997). "Psittacosis". Semin Respir Infect. 12 (1): 7–11. PMID 9097370.
- ^ Potter ME, Kaufmann AK, Plikaytis BD (February 1983). "Psittacosis in the United States, 1979". MMWR Morb. Mortal. Wkly. Rep. 32 (1): 27SS – 31SS. PMID 6621602.
- ^ "In 1929, Parrot Fever Gripped The Country". National Public Radio All Things Considered. May 31, 2009.
- ^ a b c "Winged Wisdom Pet Bird Magazine - Zoonotic (Bird-Human) Diseases: Psittacosis, Salmonellosis". Archived from the original on 2007-11-01. Retrieved 2007-12-29.
- ^ "PSITTACOSIS DISEASE - Pet Birds, Pet Parrots, Exotic Birds". Archived from the original on 2007-11-29. Retrieved 2007-12-29.
- ^ "Chemical and Biological Weapons: Possession and Programs Past and Present", James Martin Center for Nonproliferation Studies, Middlebury College, April 9, 2002, accessed November 14, 2008.
- ^ Baird, W. David (2016). Quest for Distinction: Pepperdine University in the 20th Century. Malibu: Pepperdine University Press. pp. 9–10. ISBN 978-0-9977004-0-4.
